I had the pleasure of getting to know Kevin and his lovely wife Susan when they came to Maui for their usual windsurfing adventure. I worked out with Susan while she was here and had such a great time.
Kevin and I at the time were helping rehab a dear friend and former US Ski Team member, Chris McCutcheon, an amazing athlete. I have to wonder how his knees are holding up these days Kevin?
Kevin has always been on the cutting edge of superior orthopedic technology and advances. As an athlete himself, he understands how important it is to get his patients back on the water, or on the slopes of Lake Tahoe. Patients arrive to his San Fransisco Clinic from all over the world and all get treated like first class athletes.

Kevin Stone, MD
“Many patients with healthy knees, but who are missing a portion of their meniscus, will benefit from a segmental re-growth,” Dr. Stone said.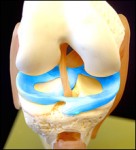
Click here to watch video of Kevin R Stone, MD. speaking about the Biological Joint Replacement: Meniscus Cartilage Replacement for Arthritis
| Collagen Meniscus Implant Opens New Vista for Knee Repair
SAN FRANCISCO, August 18, 2009 – The Stone Clinic in San Francisco reported it implanted the first commercially available meniscus templates in California this past week, opening up a whole new field of meniscus reconstruction. The implant permits segmental rebuilding of portions damaged or missing meniscus cartilage, the fibrous shock absorber of the knee.To date doctors have only been able to remove, suture repair or replace the whole meniscus with cadaver tissue. There was no method of rebuilding or regenerating missing and torn segments. Torn meniscus cartilage leads to more than 1.4 million knee arthroscopies each year in the U.S. alone. Most commonly, surgeons just remove the damage tissue leaving the knee exposed to wear and tear arthritis. The new medical procedure – collagen meniscus implant – literally allows tissue in the knee to re-grow.
 Menaflex Collegan Meniscus Implant The patients who received the first implants — one a women in her sixties, who hurt her knee ballroom dancing, a 46 year-old chiropractor, who hurt his knee weightlifting, and a 26 year-old male who hurt his knee surfing — were able to resume physical therapy on day one after surgery. “This new implant is similar to a rose trellis, acting as a guide for the new tissue growth,” said Kevin R. Stone MD, who heads the Stone Clinic. “The implant reinforces and repairs existing tissue and literally becomes part of the body. There are no artificial devices. The collagen implant is a temporary template and serves as a scaffold for tissue re-growth.” Many patients with healthy knees, but who are missing a portion of their meniscus, will benefit from a segmental re-growth, Dr. Stone said. The device is the only one engineered for patients missing meniscus tissue. It is also a great repair device for difficult-to-repair meniscus tears, such as bucket-handle tears and horizontal cleavage tears with missing volume. This new implant which Stone calls a “regeneration template” was invented by him in 1986 and used in his first human patient trial in 1990, but then took a lengthy course through the FDA. The implant marketed by ReGen Biologics Inc. and called “Menaflex” received recent press for the way the FDA handled the approval process.
Background
Twenty-five years ago, orthopedic surgeon Kevin R. Stone, MD, of The Stone Clinic in San Francisco, answered the challenge of his mentor: “If you could repair the meniscus cartilage, you would make a big contribution to people’s lives.” This week, that challenge came to fruition when Stone implanted the first commercially available collagen meniscus implant into the knees of two patients. The journey from medical challenge to medical solution has not been easy. “Early on, I realized that I could not replace the meniscus with artificial material because no artificial material was soft enough, yet durable and slick enough not to damage the opposing articular cartilage in peoples’ knees,” Stone said. “So, I sought to re-grow the meniscus and did so in 1986 by designing the first tissue engineering product in orthopedics: the collagen meniscus implant.” Stone, who won the International Society of the Knee’s Albert Trillat Young Investigator’s Award for his meniscus regeneration work in 1989 and then the American Orthopedics Society for Sports Medicine’s Cabaud Award in 1990, never imagined the process would take 22 years from invention to FDA-approval in the U.S. The implant, now marketed by ReGen Biologics, Inc., the company Stone co-founded with colleague Richard Steadman M.D, in 1989, underwent a tortuous course. The initial clinical trial in 10 patients was completed at The Stone Clinic in San Francisco in the early 1990s with successful results. Trials were repeated again with successful results, leading to a wide clinical trial in the U.S. and approval in Europe where more than 3,000 implants have already been performed. The U.S. trial and gaining FDA approval took until the end of 2008. Now, over 20 years later, Stone has the joy of implanting his device in his own patients. “We have become very skilled at complete replacement of the meniscus cartilage with allograft menisci, even in patients with arthritis,” Stone said. “However, many patients with healthy knees – but who are still missing a portion of their meniscus – will benefit from a segmental re-growth. The device is perfectly engineered for this type of procedure. It is also a great repair device for difficult-to-repair meniscus tears, such as bucket-handle tears and horizontal cleavage tears with missing volume. Stone’s medical odyssey demonstrates how long the process of invention can be, but the outcome is worth it for those willing to persevere. |
|
The Stone Clinic 3727 Buchanan Street San Francisco, CA 94123 Telephone: (415)563-3110 Website: www.stoneclinic.com
|
Our knees take a beating in all the sports we enjoy. Having a well-rounded workout program may help reduce injuries and allow you to perform the activities you enjoy.
Please call me if I can help you get your knees stronger you can last longer doing what you love!
Suzie Cooney, CPT
808-283-2121



